
Insights+: The US FDA New Drug Approvals in May 2024
Shots:
-
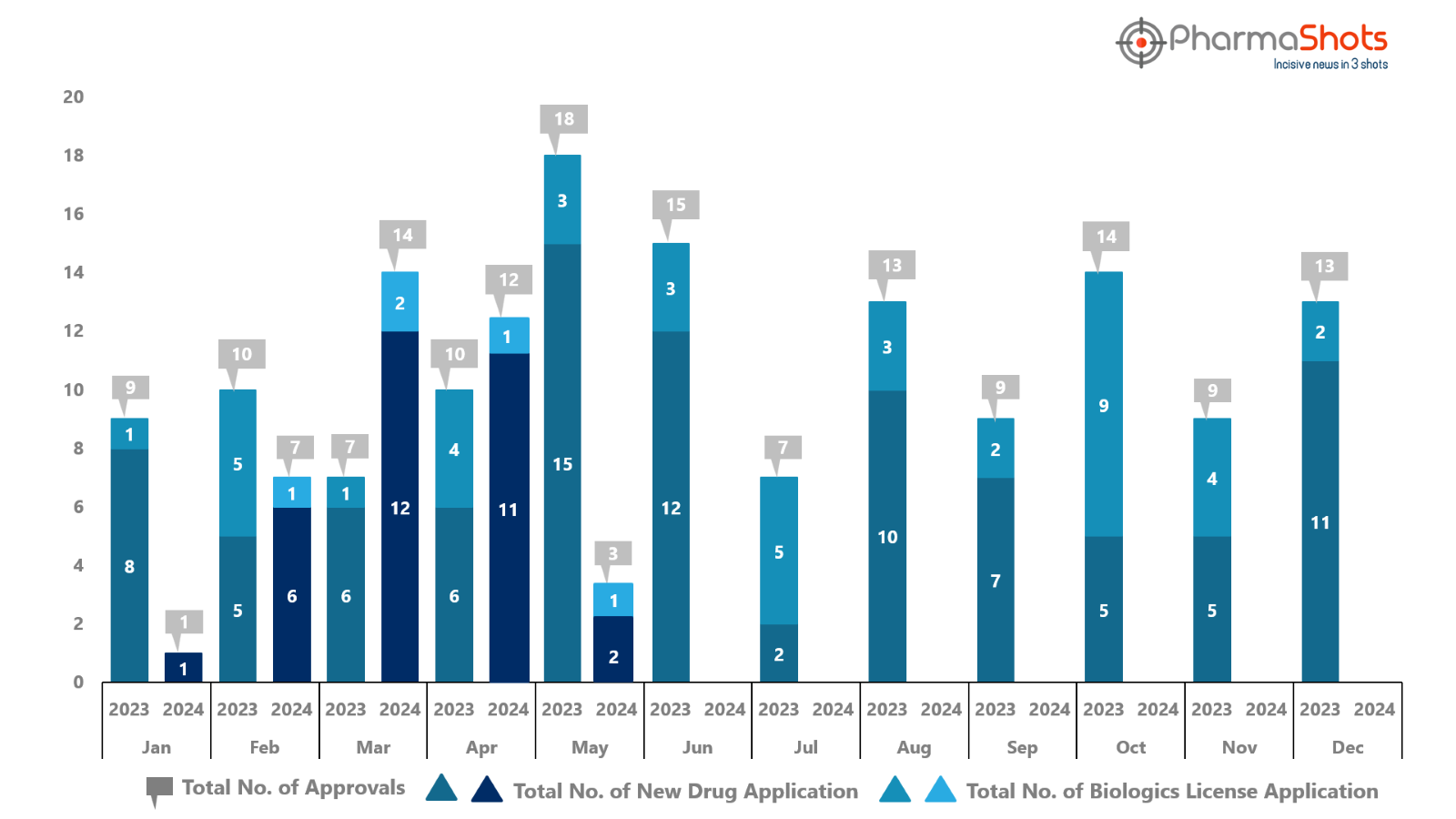
PharmaShots has compiled a list of US FDA-approved drugs in the month of May 2024
-
The US FDA approved a total of 3 new drugs including 2 new molecular entities and 1 biologic leading to treatments for patients and advances in the healthcare industry
-
The major highlighted drug was Amgen’s Imdelltra for the treatment of ES-SCLC
Active Ingredient: Mycophenolate mofetil
Approved: May 01, 2024
Company: Azurity Pharmaceuticals
Disease: Organ Rejection
-
The US FDA has approved Azurity Pharmaceuticals’ Myhibbin oral suspension with its commercial availability anticipated in Q2’24
-
Myhibbin in addition to other immunosuppressants is intended for preventing organ rejection in adults & pediatric patients (age: ≥3mos.) of allogeneic kidney, heart, or liver transplant
-
Mycophenolate mofetil, an antimetabolite immunosuppressant, works on the immune system of the body to prevent organ rejection
Active Ingredient: Tarlatamab-dlle
Approved: May 16, 2024
Company: Amgen
Disease: Extensive-Stage Small Cell Lung Cancer
-
The US FDA has granted accelerated approval to Amgen’s Imdelltra (DLL3-targeting bispecific T-cell engager) for treating extensive-stage small cell lung cancer (ES-SCLC) adults whose disease progressed post Pt-based CT. Full approval depends upon confirmatory trials
-
The approval was based on the P-II (DeLLphi-301) study assessing Imdelltra (10mg, Q2W) in SCLC patients (n=99) failing on 2 or more previous lines of treatment & administered with the 10mg Q2W dosing regimen
-
The results depicted an ORR of 40%, mDoR of 9.7mos. and mOS of 14.3mos. Further evaluation continues for complete survival data
Active Ingredient: Clonidine hydrochloride
Approved: May 24, 2024
Company: Tris Pharma
Disease: Attention Deficit Hyperactivity Disorder
-
The US FDA has granted approval to the company’s QD extended-release oral suspension of Onyda XR (clonidine hydrochloride) with nighttime dosing alone or as adj. to approved central nervous system (CNS) stimulant therapies for ADHD treatment in children (≥6yrs.). Its availability is anticipated in H2’24
-
The approval was supported by the data from studies evaluating clonidine hydrochloride extended-release tablets
-
Onyda XR is the first non-stimulant ADHD medication that has been developed using the company’s LiquiXR technology for treating ADHD
Related Post: Insights+: The US FDA New Drug Approvals in April 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














